© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
Global Heterogeneity, H:
calculation plot: FG, Kis
(see local heterogeneity h(θ) function)
Global heterogeneity plot θ(1-θ) vs. ln(p) for Fowler-Guggenheim (FG; mean-field non-specific lateral interactions) and Kiselev (Kis; specific associative interactions) compared with Langmuir equation (in the absence of heterogeneity).
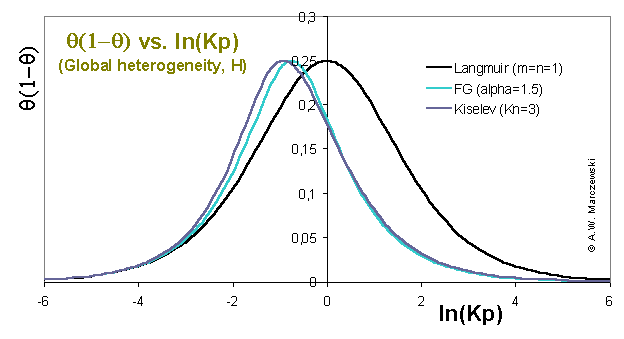
The position of the "peak" maximum corresponds to the θ = 0.5, i.e. half-filled monolayer. All plots should be compared to Langmuir isotherm line for the same average energy:
Kavg = Koexp(Eavg) where Eavg = εavg/RT
which is the reference for monolayer localized physical adsorption - if no lateral effects are present the isotherm "peak" in this plot should always be wider than the Langmuir. In the absence of heterogeneity and presence of lateral interactions the "peaks" should be narrower. (Another possiblity of comparison is shifting isotherms in such a way that their θ = 0.5 points - "peak" max. - coincide.)
Similarity of FG and Kiselev equations - with respect to the behaviour of their isotherms) is visible. FG and Kiselev isotherms also behave similarly to Langmuir for low and high coverages, i.e. their courses are identical for low coverages and their "peak" maxima and high coverage branches are shited along ln(p) towards lower pressures for higher coverages.
However - in the absence of surface heterogeneity - the corresponding "peak" widths for isotherms including lateral interaction are always lower than for Langmuir isotherm. This "narrowing" corresponds to "faster"/easier adsorption than for Langmuir - at moderate and high coverages the adsorption forces become larger (due to attractive adsorbate-adsorbate forces) and the for the same coverages the pressures may be lower than for Langmuir model. If we look just at high and low coverages it is like adsorption according to two different Langmuir isotherms - at low pressures with low equilibrium constant K, at high coverages with high equilibrium constant K.
NOTE. Replace p with c for dilute solute adsorption.
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl