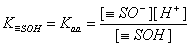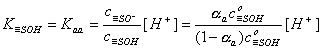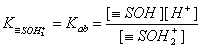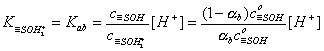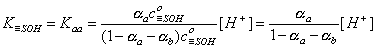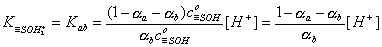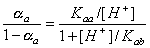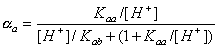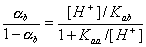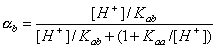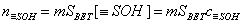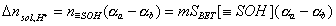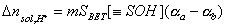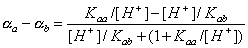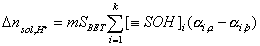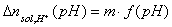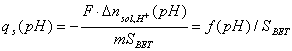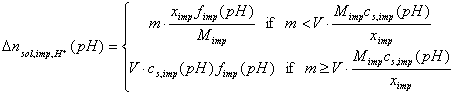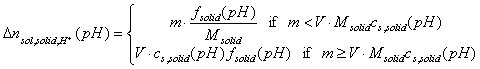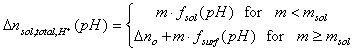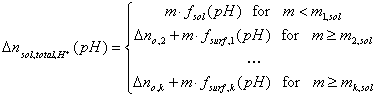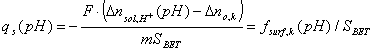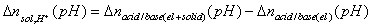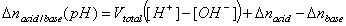© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
Reload Titration
Potentiometric titration
NOTE
Chapter based on discussions with W. Janusz and author's own experience.
See also:
W. Janusz, Chapter 4: "Electrical Double Layer at the Metal Oxide-Electrolyte Interface", in: "Interfacial Forces and Fields - Theory and Applications" (ed. Jyh-Ping Hsu), "Surfactant Science Series", vol. 85, Marcel Dekker Inc. (1999) 135-206.
W. Janusz, "Electrical Double Layer at Oxide-Solution Interfaces", in: "Encyclopedia of Surface and Colloid Science", Marcel Dekker Inc. (2002) 1687-1703.
Equations |
Model pictures |
Example |
How to calculate |
Influence of CO2
Related Data&Tools pages (
Water /
Activity /
Carbon dioxide )
Potentiometric titration (in this case versus pH-electrode) is a method, in which we titrate some system (usually aqueous solution or suspension) with specific titrant. It is possible to estimate e.g. surface charge of solid by comparing the titration of solution with solid against titration of the same solution without solid. The difference of titrant quantity between respective points characterized by the same pH value allows to estimate the influence of solid on the equilibrium. It depends on your system, whether such differences may be explained by an electric charge created on solid surface (e.g. silica, SiO2 , or alumina, Al2O3 , at low pH), or dissolution of solid (e.q. citric or oxalic acid) or both phenomena (surface charge and partial dissolution, e.g. SiO2 or Al2O3 at high pH values). Another possibility is e.g. presence of some impurities remaining after sythesis (e.g. NaOH or NH3 in porous glasses formed by precipitation from solution).
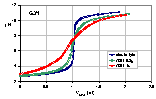 See an example of titration of lessive soil: raw titration data of 2 soil samples compared with electrolyte titration, titration proton balance and proton balance analysis taking into account surface charging and chemical reaction and/or solubility of sample.
See an example of titration of lessive soil: raw titration data of 2 soil samples compared with electrolyte titration, titration proton balance and proton balance analysis taking into account surface charging and chemical reaction and/or solubility of sample.
NOTE
The equations presented below are often simplified (compare papers above) - e.g. electrostatic factors in definitions of equilibrium functions are ignored. However, despite such simplification the derived equations (e.g. acid/base balance as a function of sample mass and pH) still describe the main features of the observed titration curves - without going into details of charge formation mechanism. All other fenomena (e.g. H+ and OH- adsorption, or adsorption of background electrolyte ions) may be in practice represented as quasichemical reactions. In order to separate the effect of specific adsorption of background electrolyte and other similar phenomena from the observed titration curves, independent parallel measurements of ion adsorption are required (e.g. radioanalytical measurements of Na+ and Cl- adsorption, ion-selective measurements of Cl-, UV/VIS spectrophotometry for organics etc.)
Charge formation
If the solid has some surface groups, they are often e.g. hydroxyl groups (often coded =SOH) being able to donate protons, accept protons or both.
For acidic groups (producing protons) with Kaa ionization constant:
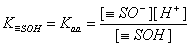
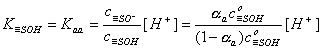
For basic groups (accepting protons) with acidic Kab ionization constant::
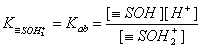
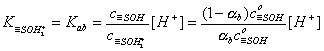
However, for amphoteric =SOH groups, one has to take into account both acidic and basic ionization:
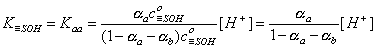
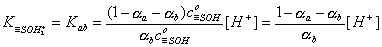
Then we obtain:
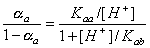 and
and
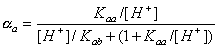
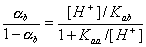 and
and
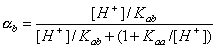
Balance of surface group forms and protons in solution
For a homogeneous solid, the amount of surface groups may be given as:
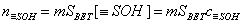
However, the amount of produced protons (if negative, protons are consumed by solid) will be proportional to the balance of acidic and basic groups:
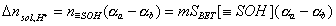
Thus, the amount of produced protons is proportional to mass of solid and - for a given kind of solid - proportionality constant depends only on solution pH:
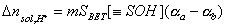
where (αa - αb) depends on pH only:
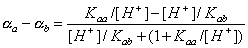
Heterogeneity of surface groups
If a solid surface contains k different groups able of acidic and basic ionization (e.g. the solid is a mixture), we may write:
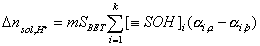
 Then, the presence of surface groups results in a change of total ionic charge in solution (excess or deficit of protons or hydroxyl ions) proportional to the amount of solid. The same will be true for any completely soluble substance (e.g. citric or oxalic acid) - in such a case, the balance of acidic/basic groups depends on substance's mass (in fact there will be no surface to deal with!). Analogously, if solid contains some amount of soluble impurities (eg. remains of strong base/acid from synthesis), the proportionality law will still hold, though the surface charge determined from such a plot will contain some systematic - sometimes very grave - error.
Then, the presence of surface groups results in a change of total ionic charge in solution (excess or deficit of protons or hydroxyl ions) proportional to the amount of solid. The same will be true for any completely soluble substance (e.g. citric or oxalic acid) - in such a case, the balance of acidic/basic groups depends on substance's mass (in fact there will be no surface to deal with!). Analogously, if solid contains some amount of soluble impurities (eg. remains of strong base/acid from synthesis), the proportionality law will still hold, though the surface charge determined from such a plot will contain some systematic - sometimes very grave - error.
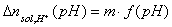
Surface charge
From the last equation we have surface charge:
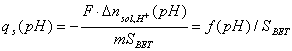
where: n - acid/base balance per sample ([mol] or [mmol]), F is Faraday constant (F=96500 C/mol), m - sample mass [g], SBET - surface area [m2/g];
qs is usually expressed as [C/m2] or µC/cm2.
Influence of partially soluble impurities and/or sample
NOTE
In all subsequent equations the following is implied:
surface charge or mass of solid do not (noticeably) decrease in the result of solubility (or chemical reaction) of impurities or solid in solution.
The above simple dependencies will be true only if no solubility limit exists (for impurities and solid). If any non-soluble solid has some weakly soluble impurities (small mass fraction ximp), and if those impurities may affect solution pH (e.g by chemical reaction with acid/base or by dissociation, fimp), then:
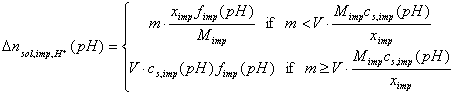
On the other hand, the solid itself may be partially soluble - depending on the pH range:
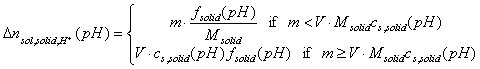
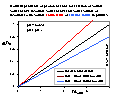
 Thus, for large mass/volume (m/V) ratios and a single type of impurities (or weakly soluble solid), for small masses of titrated solid we will observe direct proportionality (charging and dissolution). However, for larger masses of solid the slope will change and will correspond to surface charging only.
Thus, for large mass/volume (m/V) ratios and a single type of impurities (or weakly soluble solid), for small masses of titrated solid we will observe direct proportionality (charging and dissolution). However, for larger masses of solid the slope will change and will correspond to surface charging only.
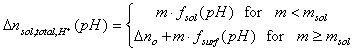
 For a larger no. of various impurities, instead of straight line from 0 point, we will se a polygon (or curve) with decreasing slope and increasing intercept.
For a larger no. of various impurities, instead of straight line from 0 point, we will se a polygon (or curve) with decreasing slope and increasing intercept.
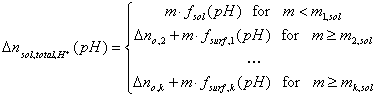
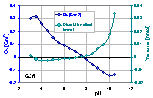 Then, the surface charge should be determined from the lowest slope (large masses), where the solution is saturated with solid and impurities:
Then, the surface charge should be determined from the lowest slope (large masses), where the solution is saturated with solid and impurities:
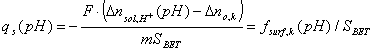
Calculation of surface charge from titration data
See also practical How to calculate ...
The Δnsol,H+ , required in order to calculate surface charge, may be calculated by comparing the balance of protons and hydroxyl ions in titration of solid with electrolyte titration:
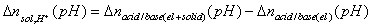
where solution acid/base balance (Vtotal is usually slightly different for electrolyte and solid titrations because various amounts of acid and base must be added in order to reach the same pH value):
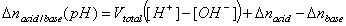
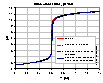
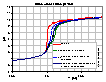
Influence of CO2 on electrolyte titration
Top
My papers
Search for papers
Main page
E-mail addresses are modified to in order to prevent spamming / mail-abuse:
in e-mail remove spaces, replace " AT@AT " by "@"
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl
Disclaimer
 See an example of titration of lessive soil: raw titration data of 2 soil samples compared with electrolyte titration, titration proton balance and proton balance analysis taking into account surface charging and chemical reaction and/or solubility of sample.
See an example of titration of lessive soil: raw titration data of 2 soil samples compared with electrolyte titration, titration proton balance and proton balance analysis taking into account surface charging and chemical reaction and/or solubility of sample.