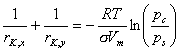 Adsorption in mesopores
Adsorption in mesopores
© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
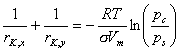 Adsorption in mesopores
Adsorption in mesopores
See an example of
surface area / pore volume analysis report
BJH scheme details and pore filling graphics
NOTE
Adsorption in mesopores according to the classical Kelvin model (below) occurs in vapours. Liquids require a different approach (e.g. DFT).
Adsorption in mesopores (usually pores of 2 - 50m - IUPAC - or sometimes 2 - 100 nm in diameter).
Typical adsorbates are N2 (nitrogen vapours at the temperature of liquid nitrogen bath at atmospheric pressure, approx. 77-78K - depending on liquid N2 purity and pressure) and vapours of benzene, C6H6 , at room (i.e. 25°C) temperature. Krypton is often used for low pressure isotherms. Other gases like CH4, CO2 are also used.
Adsorption in a so called "mesopore range" is a combination of physical adsorption on mesopore walls (described by one of theoretical or experimental isotherm equations like HJ or FHH or other - sometimes with wall curvature corrections) with physical condensation of adsorbate in pores, where meniscus reached a critical radius. It is assumed that in this "mesopore" range all micropores are already filled-up and monolayer is filled, too.
Generally, experimental isotherms measured on mesoporous solids display hysteresis loops (adsorption and desorption go along different paths) in the mesopore region. However, hysteresis is observed only when the menisci in the adsorption and desorption "paths" are different in shape/diameter. Such situation is typical for open-ended cylindrical mesopores, bottle-shaped (ink bottle), or spaces between parallel sheets (slits). If cylindrical pore is closed at one end and does not contain any narrows, the adsorption isotherm will not diplay hysteresis loop. The same is true for conical pores.
For typical adsorbates the opening/closing of hysteresis loop is found at:
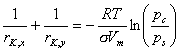 As the true pore diameter is a sum of 2 adsorbed layer thicknesses and meniscus diameter, the methods of data analysis use meniscus radii calculated from Kelvin equation (or its modifications including e.g. influence of menicus radius on adsorbate surface tension - e.g. by Dubinin) and statistical layer thickness of adsorbate (often given by Harkins-Jura (HJ) or by Halsey / Frenkel-Halsey-Hill (FHH) equations).
As the true pore diameter is a sum of 2 adsorbed layer thicknesses and meniscus diameter, the methods of data analysis use meniscus radii calculated from Kelvin equation (or its modifications including e.g. influence of menicus radius on adsorbate surface tension - e.g. by Dubinin) and statistical layer thickness of adsorbate (often given by Harkins-Jura (HJ) or by Halsey / Frenkel-Halsey-Hill (FHH) equations).
Very often in simple data analysis specific expressions for N2 adsorption are used: tHJ and tHalsey, respectively - see also above). Those simple equations (i.e. HJ and FHH) are also used in a so called t-plot method which is a simple method of determination of total micropore adsorption and external (i.e. mesopore + macropore) surface area. A better method is an αs-plot which compares adsorption data on porous solid with experimental isotherms measured on a similar in nature/chemical structure - but non-porous (or having much less micro- and mesopores) - adsorbent.
Calculations of mesopore size distribution depend on many factors:
 Barret-Joyner-Halenda (BJH)
Barret-Joyner-Halenda (BJH) 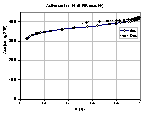 Commented example of surface area/pore volume analysis report of experimental activated carbon RIB (Norit b.v., Amsterdam, Netherlands) - adsorption/desorption isotherms of N2 at 78K measured with ASAP 2405N automatic sorption analyzer (Micromeritics, USA). Original text report (black text) includes pictures and additional explanations (blue) / comments (green) from myself.
Commented example of surface area/pore volume analysis report of experimental activated carbon RIB (Norit b.v., Amsterdam, Netherlands) - adsorption/desorption isotherms of N2 at 78K measured with ASAP 2405N automatic sorption analyzer (Micromeritics, USA). Original text report (black text) includes pictures and additional explanations (blue) / comments (green) from myself.
Top
My papers
Search for papers
Main page
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl