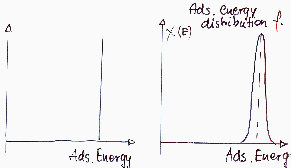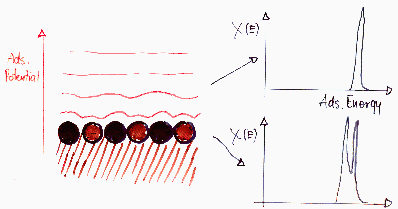© A.W.Marczewski 2002
A Practical Guide to Isotherms of ADSORPTION on Heterogeneous Surfaces
Reload Adsorption Guide
ADSORPTION:
Energetic Heterogeneity - sources
Not finished!!!
General Integral Equation
Types |
homogeneous (
mono |
bi
)
heterogeneous (
crystallinity |
defects |
groups |
composition |
pores
)
Types of heterogeneity
There are 2 main types of adsorbent heterogeneity that may be observed in adsorption isotherms:
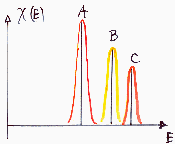
Energetic heterogeneity is a feature of entire adsorption system. It is observed when the adsorbed molecule displays varying affinity to the adsorbent surface depending on the place on the surface. Very often a term "adsorption site" is used in this context, assuming that a molecule is at least partially localized and occupies a certain area. Due to the atomic nature of matter, there are always valleys, shoulders and peaks of the potential even on the smoothest, purest, most ordered available materials (see below). Thus such small valleys of potential (caution - the potential profile depends not only on the surface but also on the adsorbate molecule!) act as localizing factor - the degree of localization depends on the depth of such potential "site" vs. the kinetic energy of the molecule on the surface (kT). One has to note that any asymmetric molecule (i.e. from the point of view of adsorption - such a one that may be adsorbed in more than one way on the surface) may also produce a distinct "heterogeneity effect" - in such a case this energetic heterogeneity is in contradiction to the most popular view of energetic heterogeneity as just a property of adsorbent surface.
From such a point of view, the structural heterogeneity (mostly porosity, but also surface defects etc. - anything that is related to the shape of the interface) - very often treated as something that may be analyzed only within the special theories of its own (e.g. theory of Dubinin-Radushkevich for micropores, BJH for mezopores) - may be regarded as merely source of energetic heterogeneity, at least for the region of low adsorption values (much below monolayer filling) or corresponding low pressures or low concentrations of the adsorbate. (Of course, for higher pressures or concentrations, such approximation is not sufficient.)
Depending on the shape/curvature of the adsorbent surface (interface), the potential acting on adsorbate is may be different - the concave surface (e.g. inside the pore near its wall) means stronger interaction with adsorbate molecule, in the case of saddle the potential may be the same as for flat surface (if both x- and y-curvature radiuses are the same with opposite signs, i.e. covex and concave), for convex surface (e.g. outside the adsorbent granule) the interactions are weaker (a very useful notion here is Hamaker constant).
Energetically homogeneous surfaces:
Many experimental isotherms display a behavior corresponding to the Langmuir isotherm.
It suggests that all adsorbate molecules adsorb with the same or almost the same energy.
Though adsorption energy reflects the properties of the adsorption system,
quite often only the properties of the surface are taken into account. Such approach may be treated
as a first approximation, though sometimes a quite good one.
In the following only the specifics of the surface will be taken into account - an advanced approach
requires the knowledge of the surface topography and molecule configuration, which is especially true in
the case of multicomponent adsorption or for larger molecules (occupied area different than the typical
size of the "surface site", i.e. smallest individually distinguished adsorption space) (see the
multicomponent adsorption, especially prediction).
Energetically homogeneous surface - identical atoms or molecules
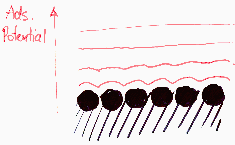
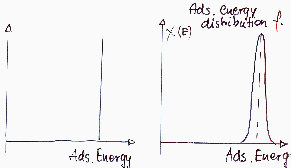
Energetically (almost) homogeneous surface - similar atoms or molecules
Surface sites may be ordered or no - homogeneity depends also on the size and kind of probe molecule (i.e. adsorbate):
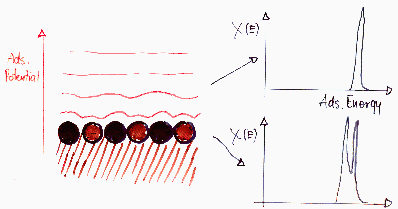
Energetically heterogeneous surfaces:
Sources of the energetic heterogeneity:
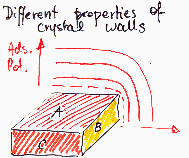
Heterogeneity - properties of crystalline walls, degree of crystallinity
Amorphous solids may behave as more or less homogenous than their crystalline forms.
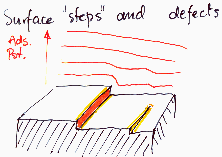
Heterogeneity - surface defects or layering steps
Unsaturated bonds may form traps for adsorbate molecules, though this efffect should depends on adsorbate properties.
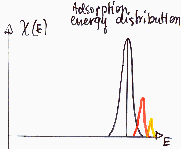
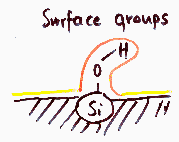
Heterogeneity - surface groups
Usually not important in the case medium and high pressure region of low temperature nitrogen adsorption used in determination of BET surface area or mesopore distribution ("white" and "black" adsorbents behave very similar).
Besides typical intermolecular - specific or non-specific interactions (van der Waals, hydrogen bond etc.), such groups may dissociate in electrolyte solution what results in dramatic change of surface properties, i.e. formation of electric charge (positive or negative) and electriostatic potential of varying magnitude. The influence of such potential on adsorption depends on liquid medium (dielectric constant, ionic strength) as well adsorbate (dissociation - cationic, anionic, amphiphilic - or no charge) and may increase or decrease apparent heterogeneity.
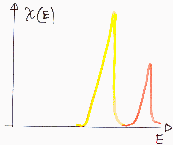
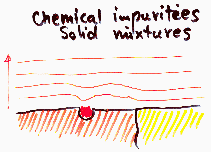
Heterogeneity - non-uniform chemical composition, impurities
The effect on adsorption depends on specific adsorbate interaction with surface. E.g. hydrophilic impurities may be very important for adsorption of water vapour on hydrophobic surface. Usually not important in the case medium and high pressure region of low temperature nitrogen adsorption used in determination of BET surface area or mesopore distribution ("white" and "black" adsorbents behave very similar).
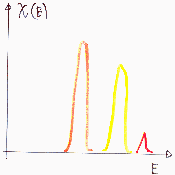
Heterogeneity - porosity, mainly meso- and microporosity
For high curvatures (small pores) the adsorption energy may be very high. Sometimes sieving effects are also important. For high pressures/concentrations multilayer adsorption and such specific effects as capillary condensation and isotherm hysteresis must be taken into account (in such situation the heterogeneity effects are masked and quite often are insignificant). Macropores are hard to distinguish from flat surface due to their low curvature (high curvature radius) and thus very small change of interaction with adsorbate (macropores may be analysed by means of mercury porosimetry, which is non-adsorption method).
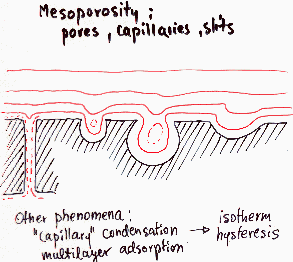
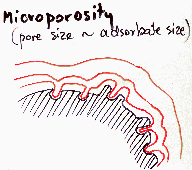

Types |
homogeneous (
mono |
bi
)
heterogeneous (
crystallinity |
defects |
groups |
composition |
pores
)
General Integral Equation
Top
My papers
Search for papers
Main page
E-mail addresses are modified to in order to prevent spamming / mail-abuse:
in e-mail remove spaces, replace " AT@AT " by "@"
Send a message to Adam.Marczewski AT@AT umcs.lublin.pl
Disclaimer